- +971 505 75 4164
- info@careonclinic.ae
- Hyatt Place Hotel, A1 Mina Road. Jumeirah. Dubai. UAE
- +971 4 280 8235
- +971 505 75 4164
- info@careonclinic.ae
- Hyatt Place Hotel, A1 Mina Road. Jumeirah. Dubai. UAE
Treatment Protocol
We are pleased to announce that we are now accepting patients under our DHA-approved treatment protocol, which includes the use of autologous fat-derived stem cells and allogeneic mesenchymal stem cells, both of which are now officially permitted for clinical use in Dubai.
Our personalized protocols are tailored to each patient’s unique clinical requirements and are built on a foundation of stringent validation, expansion, and stabilization processes. This advanced approach harnesses the therapeutic potential of both allogeneic and autologous fat-derived stem cells to help redefine the treatment of degenerative and chronic conditions.
01
Treatment Goals

Cellular Longevity Optimization
Mesenchymal stem cells (MSCs), whether sourced from a patient’s own adipose tissue (autologous) or from donated umbilical cord tissue (allogeneic), are a cornerstone of modern regenerative medicine.
Adipose-derived autologous stem cells, obtained from the patient’s fat tissue, carry a minimal risk of immune rejection and are rich in MSCs capable of differentiating into cartilage, bone, and muscle. They are widely used to promote tissue repair, reduce inflammation, and accelerate healing.
Umbilical cord-derived allogeneic MSCs, harvested from ethically donated cord tissue and processed under strict regulatory standards, possess powerful immunomodulatory and anti-inflammatory properties. These make them ideal for treating chronic and degenerative conditions, especially when autologous cells are not an option.
In parallel, their remarkable capacity for tissue repair and cellular regeneration addresses the cumulative effects of age-related degeneration. By simultaneously reducing chronic inflammation and promoting the restoration of damaged tissues, MSCs support a comprehensive strategy to slow biological aging, enhance vitality, and improve overall longevity.
Adipose-derived autologous stem cells, obtained from the patient’s fat tissue, carry a minimal risk of immune rejection and are rich in MSCs capable of differentiating into cartilage, bone, and muscle. They are widely used to promote tissue repair, reduce inflammation, and accelerate healing.
Umbilical cord-derived allogeneic MSCs, harvested from ethically donated cord tissue and processed under strict regulatory standards, possess powerful immunomodulatory and anti-inflammatory properties. These make them ideal for treating chronic and degenerative conditions, especially when autologous cells are not an option.
In parallel, their remarkable capacity for tissue repair and cellular regeneration addresses the cumulative effects of age-related degeneration. By simultaneously reducing chronic inflammation and promoting the restoration of damaged tissues, MSCs support a comprehensive strategy to slow biological aging, enhance vitality, and improve overall longevity.
Chronic Health Deterioration
Mesenchymal stem cells (MSCs), particularly those derived from human umbilical cord tissue, have demonstrated significant therapeutic potential in the management of progressive degenerative conditions.
Their efficacy is rooted in their ability to modulate immune responses and attenuate chronic inflammation—key drivers in the progression of such disorders. Through immune regulation, MSCs help establish a more balanced inflammatory environment, thereby slowing disease advancement.
Moreover, their inherent regenerative capacity enables the repair and revitalization of damaged tissues, addressing the structural decline often associated with these conditions. This is particularly evident in the field of pain management, where MSCs are emerging as a breakthrough treatment for joint-related conditions, including knee osteoarthritis and shoulder injuries. By targeting both inflammation and tissue degradation, stem cell therapy offers a minimally invasive solution that not only alleviates pain but also promotes functional recovery and joint preservation.
This integrated mechanism of immune modulation, anti-inflammatory action, and tissue regeneration positions MSCs as a promising and innovative modality in the evolving landscape of regenerative and orthopedic medicine.
Their efficacy is rooted in their ability to modulate immune responses and attenuate chronic inflammation—key drivers in the progression of such disorders. Through immune regulation, MSCs help establish a more balanced inflammatory environment, thereby slowing disease advancement.
Moreover, their inherent regenerative capacity enables the repair and revitalization of damaged tissues, addressing the structural decline often associated with these conditions. This is particularly evident in the field of pain management, where MSCs are emerging as a breakthrough treatment for joint-related conditions, including knee osteoarthritis and shoulder injuries. By targeting both inflammation and tissue degradation, stem cell therapy offers a minimally invasive solution that not only alleviates pain but also promotes functional recovery and joint preservation.
This integrated mechanism of immune modulation, anti-inflammatory action, and tissue regeneration positions MSCs as a promising and innovative modality in the evolving landscape of regenerative and orthopedic medicine.
Reduce
Inflammation
Ethically
Sourced Tissue
90% +
Viability
GMP
Cell Product
02
Protocol Overview
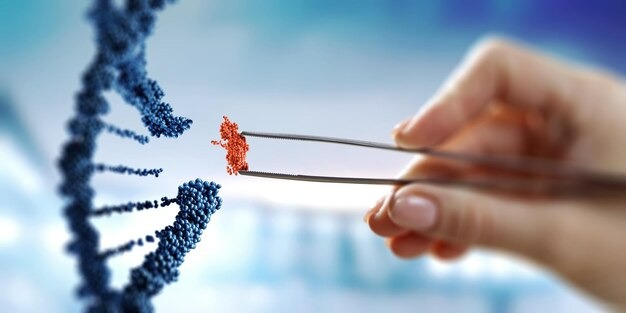
About
Our protocol begins with comprehensive laboratory testing to assess patient suitability, followed by a targeted detoxification phase aimed at reducing systemic inflammation. The core of the treatment involves the intravenous infusion of 200 million culture-expanded mesenchymal stem cells (MSCs), derived from human umbilical cord tissue. Administered over a period of 2 to 3 hours by a specialized medical team, the infusion is performed manually under controlled conditions to ensure the highest standards of safety, precision, and therapeutic efficacy.
Method
The mesenchymal stem cells (MSCs) are administered intravenously by our experienced medical team through a precisely placed IV line, typically inserted into the forearm. The infusion is conducted manually over the course of several hours, allowing for greater control and optimal cell viability, which contributes to enhanced therapeutic efficacy.
This non-invasive procedure is well-tolerated, with no associated discomfort during the administration of the cells, ensuring a safe and seamless patient experience.
This non-invasive procedure is well-tolerated, with no associated discomfort during the administration of the cells, ensuring a safe and seamless patient experience.
03
Schedule

5 Days in the City of Gold
Our clinic is conveniently located within Hyatt Place Hotel in Jumeirah, Dubai, providing a discreet and easily accessible environment for our patients. As an outpatient facility, we offer comprehensive packages that include both treatment and accommodation; however, we also provide the flexibility for patients to arrange their own travel and lodging according to their preferences.
Patients are only required to visit the clinic during scheduled appointment times, ensuring maximum convenience and autonomy throughout the treatment process.
In line with our travel protocol, we generally require patients to remain in Dubai for a minimum of four days to allow sufficient time for comprehensive evaluation, treatment, and follow-up care, supporting both optimal clinical outcomes and patient safety.
- Day 1: Arrival in Dubai
- Day 2: Laboratory Exam and Detoxification
- Day 3: Stem Cell Infusion
- Day 4: Post Treatment Procedures
- Day 5: Departure
Treatment plans at our clinic are highly individualized to address the specific health needs of each patient. While some patients may complete their treatment in as little as three days, we generally recommend a comprehensive five-day plan. This duration ensures ample time for thorough assessment, administration of multiple treatment sessions if needed, and close follow-up care. The five-day plan is designed to maximize therapeutic effectiveness and support the best possible clinical outcomes for most patients.
04
Cell Product

GMP Human Umbilical Cord Derived Mesenchymal Stem Cells (MSCs)
The mesenchymal stem cells (MSCs) utilized in our treatments are procured from the renowned Abu Dhabi Stem Cell Center, a facility recognized for its excellence in cellular therapies.
These cells undergo dynamic expansion processes to yield 200 million viable cells per treatment, with all preparation and handling conducted exclusively within the Abu Dhabi Stem Cell laboratory.
Strict adherence to Good Manufacturing Practice (GMP) standards guarantees the identity, potency, quality, and purity of our MSC product, underscoring our commitment to the highest levels of safety and therapeutic efficacy.
Strict adherence to Good Manufacturing Practice (GMP) standards guarantees the identity, potency, quality, and purity of our MSC product, underscoring our commitment to the highest levels of safety and therapeutic efficacy.
05
Validation
Viability Testing
Viability testing comprises a series of assays designed to determine the proportion of living cells within a sample, thereby evaluating the health, integrity, and functional capacity of the cells. While our partner laboratory sets a minimum viability benchmark of 85%, we consistently achieve high rates.
Maintaining high cell viability is integral to our commitment to delivering superior quality treatments. This standard not only complies with cGMP regulations but also safeguards against diminished therapeutic efficacy resulting from low viability or suboptimal cell handling.
Utilizing state-of-the-art automated cell counters, we are able to rapidly and accurately quantify mesenchymal stem cells (MSCs) with high reproducibility. These advanced systems enable precise differentiation between live and dead cells, determination of total and viable cell concentrations, and verification of counts through integrated imaging technology.
Maintaining high cell viability is integral to our commitment to delivering superior quality treatments. This standard not only complies with cGMP regulations but also safeguards against diminished therapeutic efficacy resulting from low viability or suboptimal cell handling.
Utilizing state-of-the-art automated cell counters, we are able to rapidly and accurately quantify mesenchymal stem cells (MSCs) with high reproducibility. These advanced systems enable precise differentiation between live and dead cells, determination of total and viable cell concentrations, and verification of counts through integrated imaging technology.
Immune Priviledged
Human umbilical cord tissue-derived mesenchymal stem cells (hUC-MSCs) possess immunologically evasive characteristics that render them well-suited for allogeneic transplantation without eliciting adverse immune responses.
This immune tolerance primarily arises from their minimal expression of major histocompatibility complex (MHC) class I molecules and the complete absence of MHC class II molecules on their surface, effectively enabling these cells to evade detection by CD4+ T lymphocytes and thereby preventing T cell activation.
Importantly, MSCs do not transfer or integrate their genetic material into recipient cells. Their transient engraftment, absence of genomic incorporation of engineered transgenes, and reliance on paracrine signaling mechanisms collectively indicate that horizontal DNA transfer does not occur during MSC therapy.
This immune tolerance primarily arises from their minimal expression of major histocompatibility complex (MHC) class I molecules and the complete absence of MHC class II molecules on their surface, effectively enabling these cells to evade detection by CD4+ T lymphocytes and thereby preventing T cell activation.
Importantly, MSCs do not transfer or integrate their genetic material into recipient cells. Their transient engraftment, absence of genomic incorporation of engineered transgenes, and reliance on paracrine signaling mechanisms collectively indicate that horizontal DNA transfer does not occur during MSC therapy.
06
Efficacy Data
Results
Clinical outcomes are evaluated through quantitative analysis of specific inflammatory biomarkers, complemented by subjective insights gathered from PAR-Q vitality questionnaires. While patient response is influenced by a wide range of intrinsic biological factors, long-term efficacy is also closely linked to individual behavior and lifestyle choices.
Patients who maintain an active lifestyle, adhere to an anti-inflammatory diet, and limit the use of alcohol, tobacco, and caffeine often experience sustained therapeutic benefits lasting four years or more, depending on the underlying condition.
On average, patients report minimal regression up to 48 months post-infusion, with very few returning to their pre-treatment baseline. In compliance with IRB requirements, our patient management team conducts follow-up evaluations every three months for a period of 24 months post-treatment to monitor outcomes and gather long-term efficacy data.
Patients who maintain an active lifestyle, adhere to an anti-inflammatory diet, and limit the use of alcohol, tobacco, and caffeine often experience sustained therapeutic benefits lasting four years or more, depending on the underlying condition.
On average, patients report minimal regression up to 48 months post-infusion, with very few returning to their pre-treatment baseline. In compliance with IRB requirements, our patient management team conducts follow-up evaluations every three months for a period of 24 months post-treatment to monitor outcomes and gather long-term efficacy data.
Expected Improvement Timeline
Participants may begin to exhibit measurable clinical improvements within 3 to 6 months post-infusion, typically reflected by a significant reduction in systemic inflammatory markers and enhanced vitality, as assessed through structured follow-up questionnaires.
That said, many patients report noticeable improvements in quality of life and a reduction in symptoms within the first few weeks following treatment. It is important to note that individual responses may vary, as outcomes are influenced by a range of personal biological and lifestyle factors.
That said, many patients report noticeable improvements in quality of life and a reduction in symptoms within the first few weeks following treatment. It is important to note that individual responses may vary, as outcomes are influenced by a range of personal biological and lifestyle factors.
Overall Success Rate
As measured by PARQ vitality questionnaire
0
%
Symptom Management
3/Month Average Improvement
0
%
Energy Levels
6/Month Average Improvement
0
%

